Background: Hematopoietic stem cell transplantation (HSCT) is the only cure for acute myeloid leukemia (AML), but relapse is the most important cause of transplant failure. Patients with remission but measurable residual disease (MRD) positive have a higher probability of hematologic relapse. The MRD-directed preemptive treatments can spare some patients in remission from further therapy. The conventional first-line preemptive treatment has been ineffective in some patients, so it's necessary to find a new preemptive treatment to prevent relapse and improve the prognosis. Our study aimed to evaluate the efficacy and safety of selinexor-containing regimens in the preemptive treatment of MRD positive in post-transplant patients with AML or myelodysplastic syndrome (MDS).
Methods: The study was conducted in Tongji Hospital from March 2022 to May 2023. The inclusion criteria of post-transplant AML/MDS patients included the followings: (a) Persistent MRD positive. (b) MRD turned negative after first-line preemptive treatment but was positive again. (c) Patients at high risk for hematologic relapse after transplantation; (d) AML-M4/5. The end of the follow-up was in June 2023. Bone marrow aspiration was assessed for MRD using multiparameter flow cytometry (MFC) combined with real-time quantitative polymerase chain reaction (RT-qPCR) as follows: 1) MRD assessed by MFC: MRD negativity was defined as <0.1%. 2) MRD assessed by RT-qPCR: Abnormal genes associated with prognosis were used for molecular MRD detection. 3) WT1 was used for molecular MRD assessment if no molecular marker was available at diagnosis. MRD negativity was defined as <0.6%.
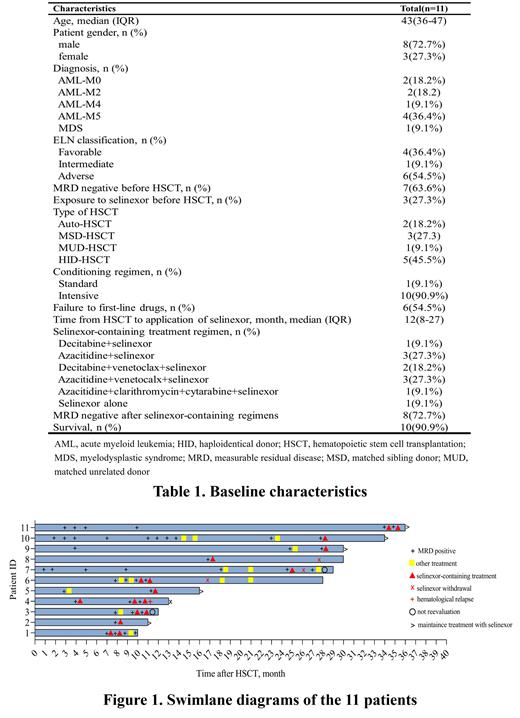
Results: A total of 11 patients were included in the study. There were 10 patients with AML and one patient with MDS, with a median age of 43 years old. Two patients had received autologous HSCT, nine patients had received allogeneic HSCT, of which two matched sibling donor, one matched unrelated donor, and six were haploidentical donor.
All patients were WT1 positive and two of the cases were both WT1 and MFC positive. Seven patients with post-transplant MRD positive were treated with selinexor-containing regimens after first-line preemptive therapy (azacytidine, azacytidine combined with venetoclax, decitabine combined with venetoclax, etc.) failed or MRD positive again. The remaining four patients were treated directly with selinexor-containing regimens for personal reasons or with a diagnosis of AML-M4/5 may be resistant to venetoclax.
The selinexor-containing regimens used in this study were either selinexor (35mg/m 2 biw) alone or in combination with other drugs (decitabine, azacytidine, venetoclax, low-dose cytarabine, and CAG (cytarabine, aclarubicin, G-CSF) regimen). MRD was evaluated after two weeks of the selinexor-containing regimens. And selinexor 20 to 40 mg per week was used as maintenance therapy after MRD converted to negative. Eight patients (72.7%) successfully cleared the MRD. Of the three patients who didn't clear the MRD, two patients successfully converted to negative after continuing the selinexor-containing regimens. It is worth mentioning that 7 of the 11 patients were treated with the selinexor-containing regimens after first-linepreemptive treatment failure, and six of them had successfully cleared the MRD (71.4%). At the end of follow-up, 10 (90.9%) patients survived and one (9.1%) died due to disease progression.
In terms of hematological adverse events (AEs), grade 3-4 neutropenia occurred in nine (81.8%) patients, the median duration was 2 days (IQR 1,4). Grade 3-4 thrombocytopenia occurred in three (27.3%) patients, and the median duration was 5 days (range 4-7). Although the number of people with grade 3-4 neutropenia was large, the duration was short after using granulocyte colony-stimulating factor, and no patient developed a severe infection. Most of the non-hematological AEs were mild, including nausea (100.0%), fatigue (63.6%), hyponatremia (58.3%), and vomiting (45.5%). The AEs can be alleviated after symptomatic treatment and no patient discontinued treatment.
Conclusion: Selinexor-containing regimens have been shown to be effective and well tolerated in post-transplant AML/MDS patients with MRD-positive, including those who have failed first-line preemptive treatment.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Patients with remission but measurable residual disease(MRD) positive have a higher probability of hematologic relapse after hematopoietic stem cell transplantation. For patients who were ineffective with conventional first-line preemptive treatment. it's necessary to find a new one to prevent relapse and improve the prognosis. Preclinical studies showed that selinexor has potent cytotoxic activity in AML cell lines and can play a synergistic role with different mechanisms of drugs. At present, there are many studies on selinexor in combination with various standards of care or intensive chemotherapy regimens for the treatment of R/R AML which have demonstrated promising results. In conclusion, selinexor-based regimens could be a choice for post-transplant AML/MDS patients with MRD-positive, especially for those who have failed first-line preemptive treatment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal